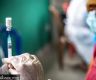
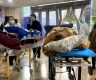
mRNA vaccine photo:Web
China's first production base for an mRNA vaccine against COVID-19, which uses domestically produced core raw materials and equipment, is expected to be put into use in October. Experts said that this proves China has grasped core mRNA vaccine technology, and it will boost the country's mass vaccination drive.
Jointly developed by the Academy of Military Medical Sciences, Suzhou Abogen and Yunnan Walvax Biotechnology Co, the mRNA vaccine called ARCoVax is expected to go into mass production in the base in Yuxi, Southwest China's Yunnan Province next month.
With an investment of 520 million yuan ($80 million), the plant, China's first production base for mRNA vaccine, has the capacity to produce 200 million doses annually.
Compared with mRNA vaccines developed by the US and Germany, the domestic mRNA vaccine is much safer as the selection of the vaccine antigen target is more precise and the neutralizing antibodies induced are higher, media reports said.
The storage cost of this vaccine is lower than those from overseas as it adopts a single injection in one package and could be stored at room temperature for a week or at 4 C for a long time, making it easier to use.
These factors make this domestic ARCoVax product stand out from Western-dominated mRNA vaccines made by pharmaceutical giants Pfizer-BioNTech and Moderna, which require much lower temperatures and more rigorous temperature controls, said experts.
The vaccine will be able to meet demand once it is put into production, as core raw materials and equipment for the Chinese mRNA vaccines have been made domestically.
A Beijing-based immunologist told the Global Times on Thursday that the domestically produced mRNA vaccine demonstrates China's huge improvements in mRNA technology and the country's leading position in biotechnology.
Such technology could be used in other biomedical fields such as oncotherapy and gene defect treatment, the immunologist said.
On Thursday, the Chinese Center for Disease Control and Prevention confirmed that domestic vaccine makers of both the mRNA and adenovirus types have launched research and development into how their vaccines could better deal with variants such as Delta and Beta.
ARCoVax has been approved to initiate its late-stage clinical trials in Mexico and Indonesia by local health authorities, Yunnan Walvax Biotechnology Co announced on August 31.
China's vaccine options will be enriched with the mixed use of different types - inactivated, mRNA or adenovirus, experts noted.
The immunologist believed the first mRNA vaccine against COVID-19 will boost the country's mass vaccination drive as the possibility of China using an mRNA vaccine as a booster shot cannot be ruled out.